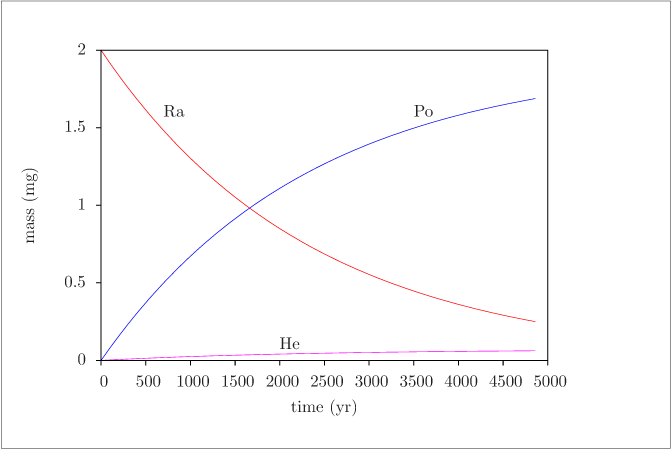
Figure 5 (page 7):
Concentrations of species in Reactions \ref {rxn:radio1}--\ref {rxn:radio2} versus time.
Code for Figure 5
Text of the GNU GPL.
main.m
clear('all'); close('all');
% solves the radium -> radon -> polonium
% transient decay
%
% jbr, 8/30/2007
%
global k1 k2
t1half = 1620; % yr
t2half = 3.83/365; % yr
samplemass = 2; % mg
% molecular weights g/mol
MA = 226; % radium
MB = 222; % radon
MC = 218; % polonium
MD = 4; % alpha particle
% initial conditions in moles per sample volume
nA0 = samplemass/MA;
nB0 = 0;
nC0 = 0;
nD0 = 0;
% rate constants
k1 = log(2)/t1half;
k2 = log(2)/t2half;
% times for output
time = linspace(0, 3*t1half, 100)';
x0 = [nA0; nB0; nC0; nD0];
[tout, x] = ode15s (@rates, time, x0);
% convert to mass per sample volume
% scale each column of x (species) by its mol wt
xmass = x*diag([MA, MB, MC, MD]);
figure(1);
plot(tout, xmass)
figure(2);
% clip off first time point, log(0) undefined
semilogy(tout(2:end), xmass(2:end,:))
table = [tout, xmass];
rates.m
function rhs = rates(t, x)
global k1 k2
na = x(1);
nb = x(2);
nc = x(3);
nd = x(4);
r1 = k1*na;
r2 = k2*nb;
rhs = [-r1; r1 - r2; r2; r1 + r2];
